Powder Vaccine Production
Introduction
Since 2020, COVID-19 has caused a global pandemic, causing fear of infection and social turmoil around the world. Governments, academia, and private businesses have been working day and night on vaccines and other treatments, leading to the development and approval of a handful of vaccines, which have then been administered to people around the world as part of a rapid worldwide immunization program. However, the developed vaccines are formulated as liquids that require either low or ultra-low temperature storage and transportation, causing enormous strain on the existing cold supply chain. Those vaccines which are frozen must also be thawed before administration and cannot be refrozen once thawed. Problems such as broken freezers, causing the need for immediate vaccine administration or the wastage of the vaccine, are abundant.
Pushing the existing supply chain beyond its limits has led to rising costs and shortages throughout the world. Furthermore, the existing cold chain is concentrated in developed countries, restricting the supply of vaccines to tropical regions and developing countries, resulting in unequal distribution and low vaccination rates.
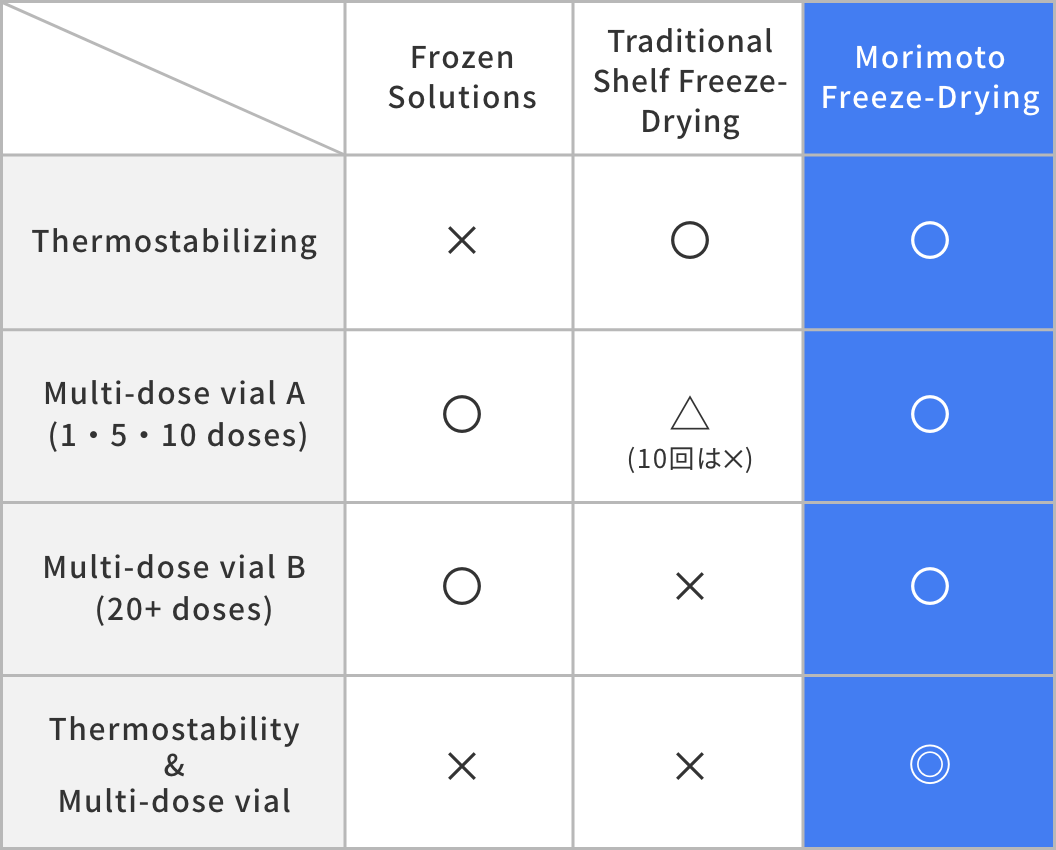
All of the issues listed above interfere with the goal of rapid worldwide immunization. As a solution to these problems, the utilization of MII's continuous lyophilization (freeze-drying) technology for the development of next-generation room temperature stable COVID-19 vaccine formulations is proposed.
High Efficiency
Vaccines are considered to be our best chance for beating COVID-19, and governments are depending on them to lead to economic recovery. The yearly production of COVID-19 vaccines is expected to reach 8.4 billion doses by the end of 2021, 41.3 billion doses in 2022, and 42.7 billion doses in 2023. But to quickly bring about the end of the pandemic, the pharmaceutical industry needs to ramp up production even faster, aiming for unprecedented production quantities. Utilization of traditional batch lyophilization technology only will not be sufficient. To meet these tremendous needs, continuous production technology must be adopted because it is up to 10 times more efficient than traditional methods.
Because of its high efficiency, continuous manufacturing with real-time monitoring is widely recognized as a superior manufacturing technology. And thus, regulatory agencies, such as the FDA and PMDA, are extremely supportive and awarding grants to spur innovation, as well as granting priority to processes utilizing this technology during drug applications.
Better Distribution
COVID-19 has caused over 5 million deaths worldwide and caused great harm to economies around the world, in some areas rivaling the damage caused by the global financial crisis. This harm was especially exacerbated in emerging countries and low-income countries. Additionally the distribution of vaccines and other treatments has been far from equal. Mid and high-income countries have received a large share of the manufactured vaccines; in low-income countries, only about 1.4% of the people have received one or more doses (as of mid-2021).
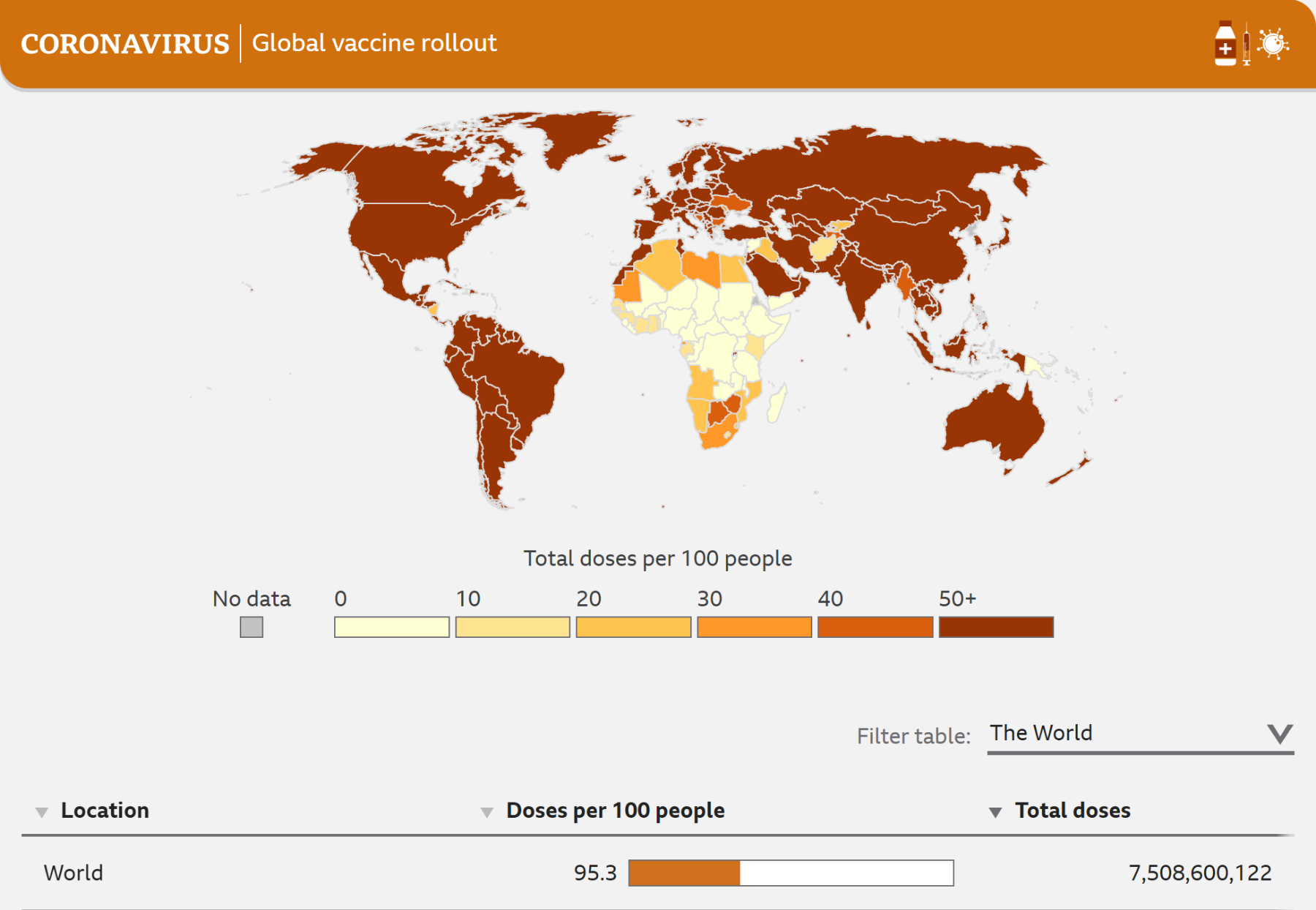
Source: https://www.bbc.com/news/world-56237778
While procurement issues exist, one of the major factors causing problems with vaccine distribution is the lack of cold chain infrastructure in the developing world. Investment in the cold chain is necessary and will help alleviate these issues. However, to truly solve the problem, the development of next-generation thermostable vaccines is needed.
Reduces Costs and Environmental Damage
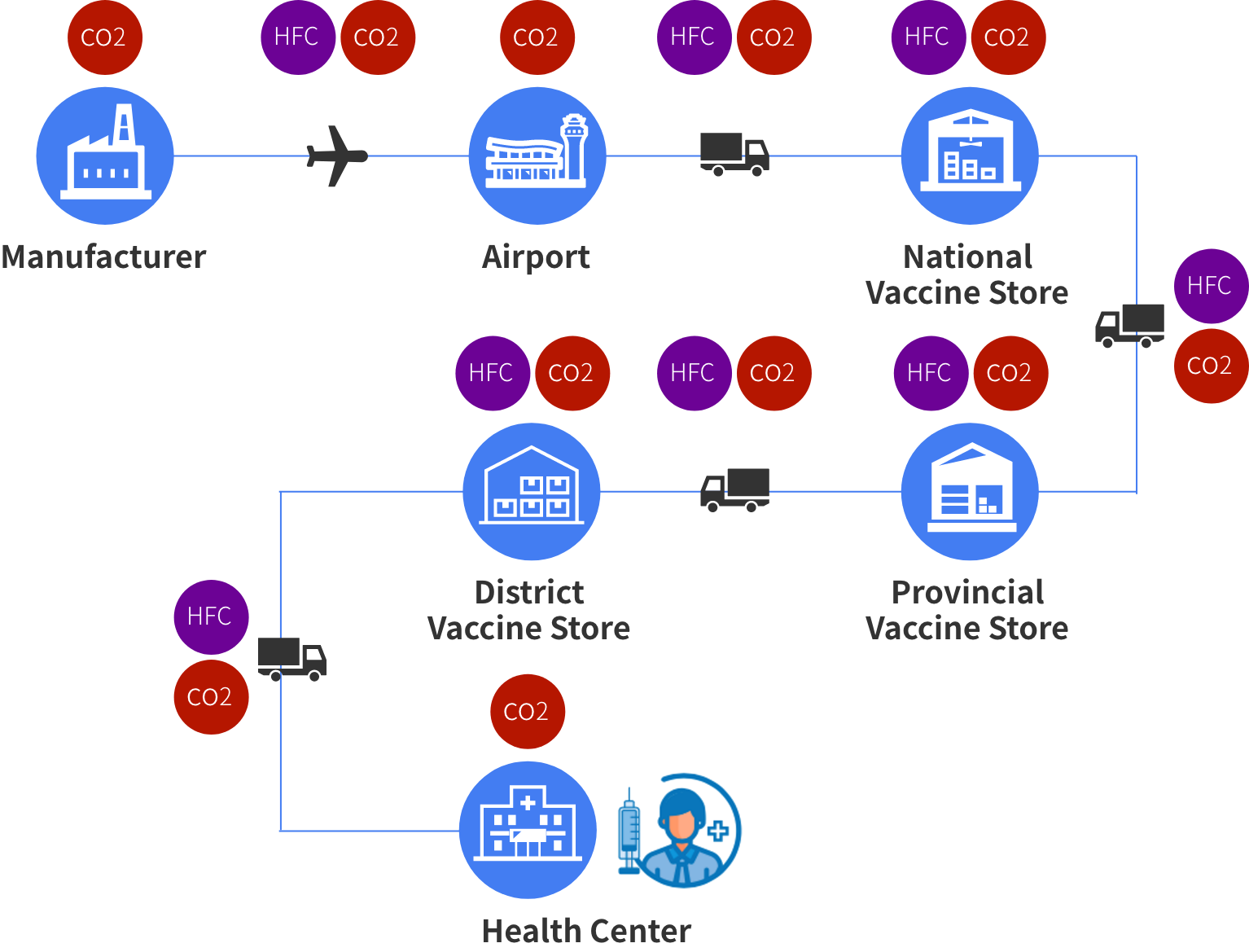
Liquid vaccines require a cold supply chain to preserve stability, in some cases ultra-cold temperatures down to -70℃ may be required. This requires cold shipment and specialized shipping containers, which add significant shipping costs.
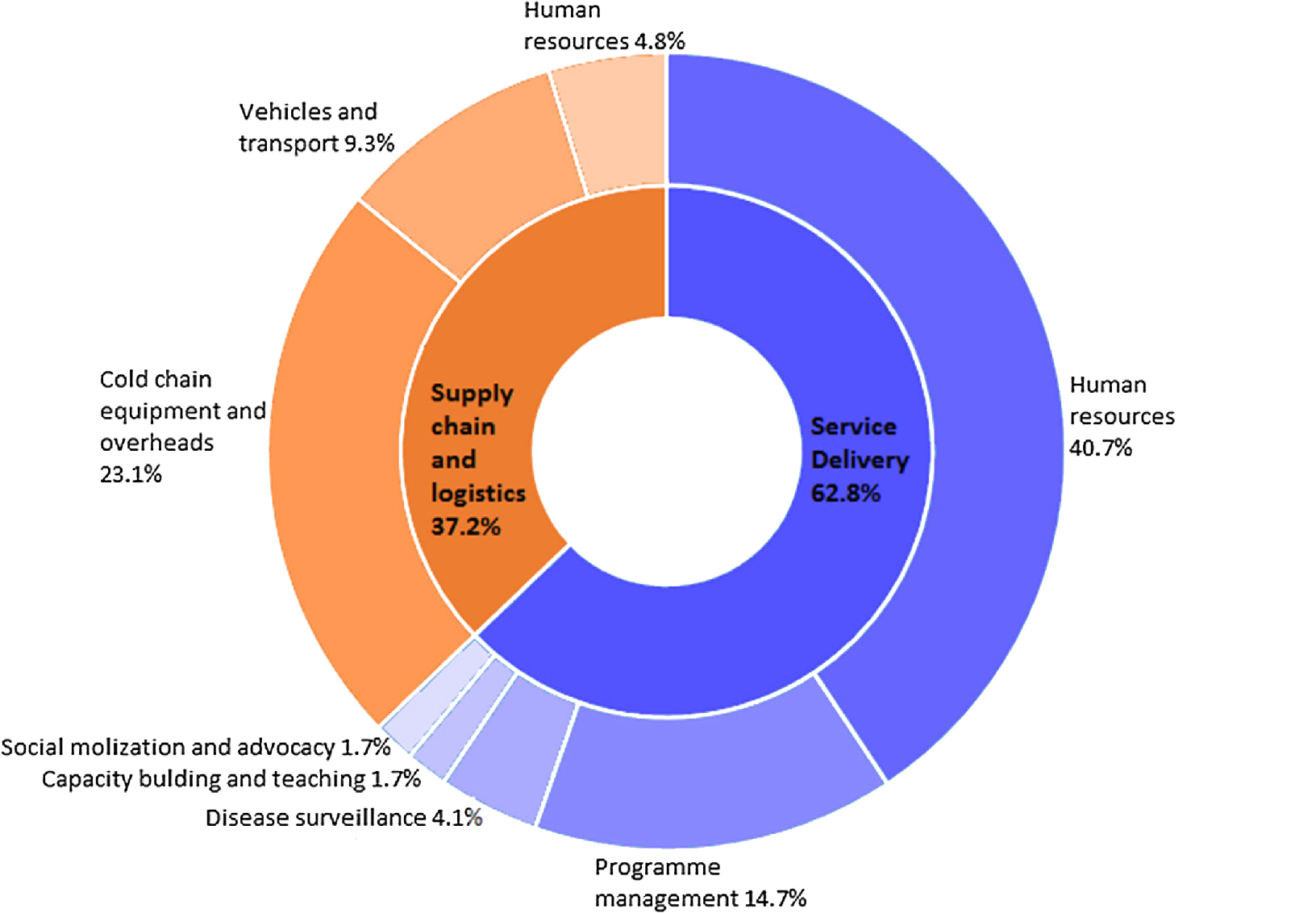
Source: Challenges for nationwide vaccine delivery in Africa
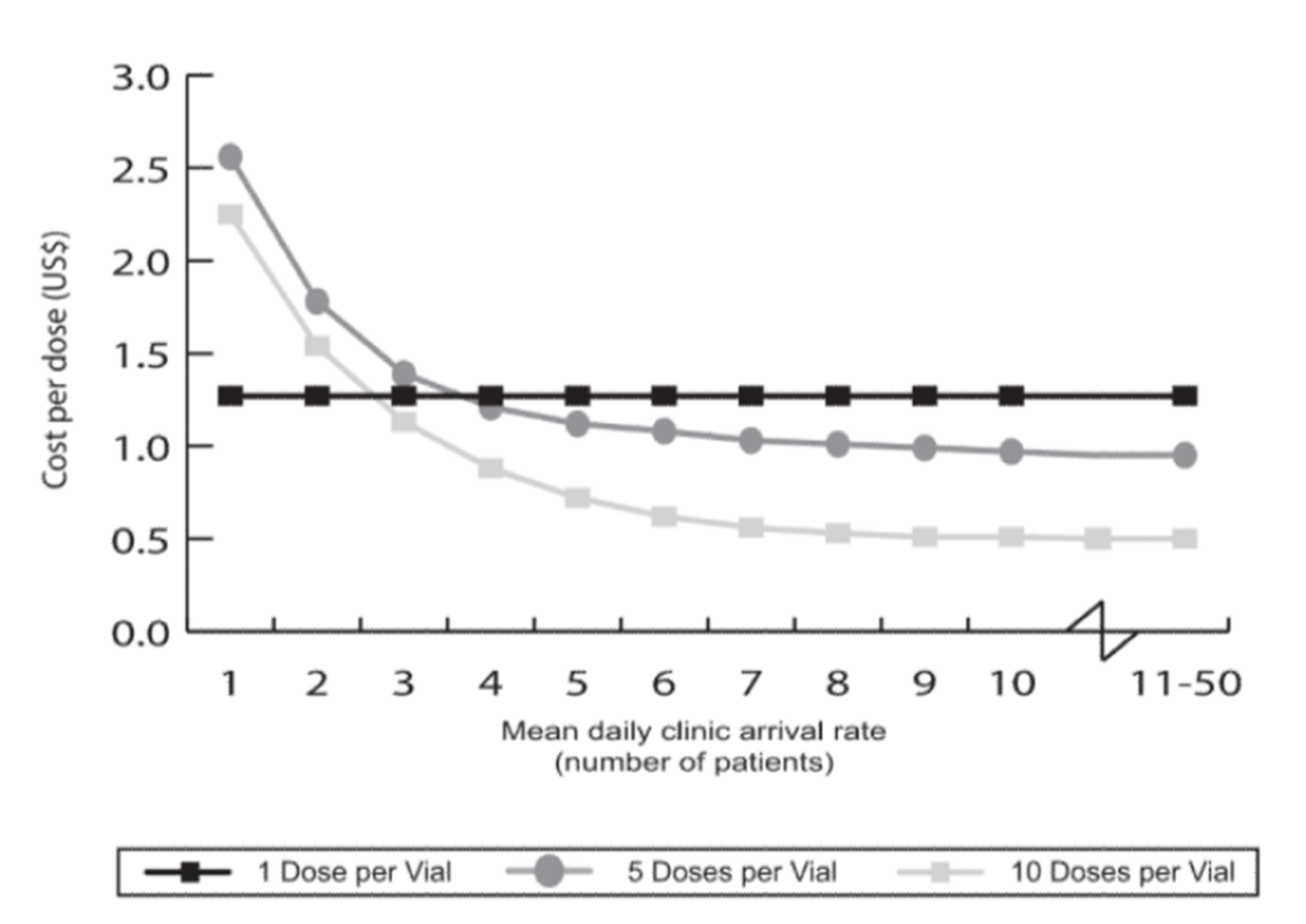
After delivery, liquid vaccines need to be refrigerated or frozen until use (storage requirements vary depending on the vaccine). Unfortunately, not all facilities have freezers that can reach the necessary conditions for storage. Cold storage equipment and its related maintenance add significantly to overall costs (on the order of 23%). Before administration, frozen vaccines must be thawed, and once thawed the vaccine cannot be refrozen. If refrigeration equipment breaks down, all vaccines contained within must be either administered in a short period or discarded. Vaccine wastage comprises a significant amount of the total cost of vaccine administration. Having a simple powder vaccine that can be prepared on demand simplifies storage and administration along with reducing equipment, facility, and staffing costs.
Regarding environmental concerns, shipping and storage via a cold chain result in both direct emissions and indirect emissions that are harmful to the environment. Direct emissions come from the burning of fossil fuels for transportation and releases of refrigerant gases from refrigeration equipment. Indirect emissions are those that are emitted from the burning of fossil fuels for electricity generation for cold storage equipment. By eliminating or reducing the need for a cold supply chain and cold storage, both direct and indirect emissions are drastically reduced. This reduction can be achieved via the use of room temperature stable powdered vaccines.
Adaptable Technology Enabling Rapid Response
to Future Pandemics
Our continuous spray lyophilization technology enables flexible production and fast responses to changing demand. Shelf freeze-drying, on the other hand, is validated for batch size and cannot be changed based on short-term needs. While shelf freeze-drying has a long history of use in the pharmaceutical industry, the reality is that quickly responding to drug demand and shortages is becoming a necessity. The COVID-19 pandemic has made this abundantly clear. There is a need for a much faster transition from small-scale clinical manufacturing to full-scale production in order to bring a quick end to future pandemics.
Another advantage of the bulk continuous production of drugs is that it allows the filling of different product types (vials, syringes, inhalation systems, etc.), further enhancing its utility. Continuous technology moves pharmaceutical production towards the future, where more and more drugs will be produced on demand.